ARTICLE AD
A study on the brains of mice has identified a neurological relay race unfolding at meal time, dividing activity into four separate phases that ensure the animals don't eat too much or too little.
While the research doesn't identify the exact physiological cues that define each act, the findings hint at a complex interplay of rewards and inhibitions that start with the first tasty morsel we pop into our mouth and end with the final bite.
Applied to human brains, the discovery could help explain some cases of eating disorders and open the way to new forms of treatment.
Researchers from the University of Erlangen-Nuremberg (FAU) and the University of Cologne in Germany looked at the neural firing rates in the brain's lateral hypothalamus, a region that plays a central role in regulating innate behaviors involving feeding, expoloring, and socializing in both mice and humans.
The team identified four distinct sets of neurons that light up one after the other, arguably running a series of checks to regulate energy intake and to signal when enough is enough in terms of eating.
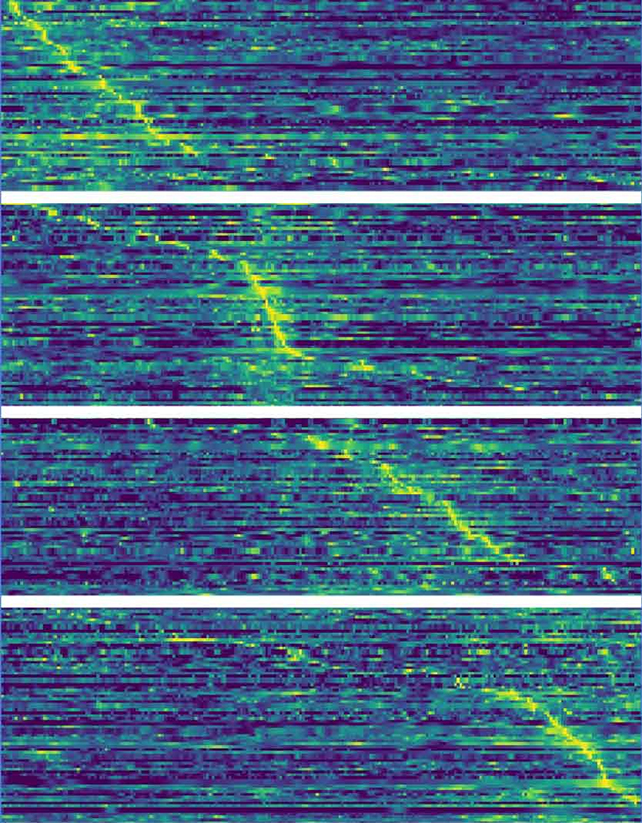 The four stages of eating, shown by the brighter neurons. (Altafi et al, Journal of Neuroscience, 2024)
The four stages of eating, shown by the brighter neurons. (Altafi et al, Journal of Neuroscience, 2024)"When we eat, we quickly switch from what we call 'appetitive' behavior to 'consummatory' behavior," says neuroscientist Alexey Ponomarenko from FAU.
"We know little about how the brain controls the duration of this consummatory phase. It should neither be too long nor too short so that we receive the right amount of energy."
The researchers used artificial intelligence algorithms to identify which neurons were active and when, based on readings taken from electrode implants. This revealed key neurons in the lateral hypothalamus collectively oscillating at frequencies that varied depending on the activity being undertaken.
Four distinct groups of neurons firing in sequence were identified by the shared frequency, assisting their ability to share information while feeding.
"We were now able to show that the teams of neurons involved in food intake all communicate on the same frequencies," says Ponomarenko.
"In contrast, groups of neurons responsible for other behaviors – such as exploring the environment or social interaction – prefer to communicate on a different channel."
Feeding involves a variety of cues, combining 'feel-good' sensations that encourage us to continue eating with signs we've had enough. It's not clear what cues trigger which sets neurons, but the researchers think they could collect and transmit physiological information on the stomach's capacity, blood sugar levels, and fluctuations in hunger hormone levels.
All of this needs to be verified in humans of course, but the similarities between mouse and human physiology mean it's likely similar activity is occurring in our own brains, letting us know when feeding time is (and isn't).
Next, the researchers want to see if this neuron relay race can be manually manipulated using light in a method known as optogenetics. The team expects a more thorough analysis of this brain circuitry could reveal new insights into eating disorders – conditions that directly affect an individual's daily feeding activities.
"In mice, the oscillatory behavior of neurons can be influenced even more directly by optogenetic manipulations," says Ponomarenko. "We are now planning a follow-up study to investigate how this affects their feeding behavior."
The research has been published in the Journal of Neuroscience.

 1 month ago
16
1 month ago
16 

