ARTICLE AD
Scientists have revealed in unprecedented detail how obesity affects mitochondria in mice, causing the cellular powerhouses to fragment into smaller pieces.
Mitochondria are famous as "powerhouses of the cell," given their crucial role in generating energy. Yet the vital work of these organelles is often impaired in people with obesity, for reasons that remain unclear.
And while impaired mitochondria are probably bad news, it's also unclear how that might influence obesity or the additional health problems it can cause.
In their new study, an international team of researchers found that when they fed mice a high-fat diet, mitochondria within the mice's fat cells broke apart into smaller mitochondria, which had a reduced capacity for burning fat.
They also discovered this process is governed by just one gene. When they deleted that gene from their test subjects, the mice avoided excess weight gain – even when fed the same high-fat diet that wreaked havoc in other mice.
"Caloric overload from overeating can lead to weight gain and also triggers a metabolic cascade that reduces energy burning, making obesity even worse," says University of California cell biologist Alan Saltiel.
"The gene we identified is a critical part of that transition from healthy weight to obesity."
Worldwide obesity has nearly tripled in the past 50 years, resulting in a major public health crisis in countries all over the planet. Along with the direct effects of obesity come many potentially serious health complications, including diabetes, heart disease, and cancer, among others.
Obesity is an excessive accumulation of fat in the body, which is commonly stored in adipose tissue. As Saltiel and team note, adipose tissue normally serves valuable mechanical and metabolic roles in the body, like cushioning organs and releasing cellular signaling molecules.
In some people with obesity, however, fat cells can become less effective at burning energy, potentially making it even harder to lose weight. Yet the origins of this metabolic anomaly remain mysterious, inspiring the team to dig deeper for answers.
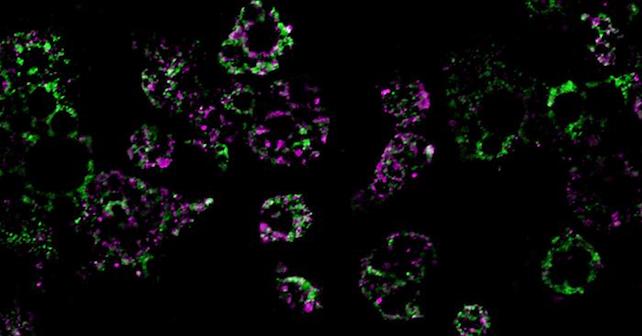 These colored streaks are mitochondrial networks within fat cells. Researchers found that a high-fat diet dismantles mitochondria, resulting in weight gain. (UC San Diego Health Sciences)
These colored streaks are mitochondrial networks within fat cells. Researchers found that a high-fat diet dismantles mitochondria, resulting in weight gain. (UC San Diego Health Sciences)Not only did their study link a high-fat diet with fragmentation of mitochondria in the mice's fat cells – resulting in mini-mitochondria that couldn't burn fat as effectively – but they also found this process is controlled by a single molecule, known as RalA.
RalA is a multipurpose molecule, the researchers note, and one of its roles is to break down mitochondria when they malfunction. But if RalA is overactive, their study suggests, it might interfere with the regular work of mitochondria, thus leading to a metabolic cascade.
"In essence, chronic activation of RalA appears to play a critical role in suppressing energy expenditure in obese adipose tissue," Saltiel says.
"By understanding this mechanism, we're one step closer to developing targeted therapies that could address weight gain and associated metabolic dysfunctions by increasing fat burning."
The researchers demonstrated the effect RalA can have by deleting its associated gene in some mice, then feeding them a high-fat diet identical to that eaten by other mice still carrying the gene.
Mice without the gene avoided the diet-induced weight gain that befell their control-group counterparts, the researchers report.
This study was conducted on mice, so it's worth noting that more research will be needed to reveal whether this applies to humans. The authors did notice that some RaIA-influenced proteins in mice are similar to human proteins associated with obesity and insulin resistance.
"The direct comparison between the fundamental biology we've discovered and real clinical outcomes underscores the relevance of the findings to humans and suggests we may be able to help treat or prevent obesity by targeting the RalA pathway with new therapies," Saltiel says.
"We're only just beginning to understand the complex metabolism of this disease," he adds, "but the future possibilities are exciting."
The study was published in Nature Metabolism.

 1 year ago
83
1 year ago
83 

