ARTICLE AD
The protein MYC is part of healthy cell activity, but when cancer cells develop, it goes haywire – going outside of its normal, carefully controlled role and helping cancer to spread. Now, scientists may have found a way to stop this from happening.
Part of the problem in reining in MYC is that it's a shapeless protein, one that doesn't really have a structure that can be targeted. That makes it difficult for drugs to effectively identify MYC and keep it behaving normally.
However, a team from the University of California, Riverside (UCR) has been able to develop a peptide compound that can bind or interact with MYC and help get it back under control.
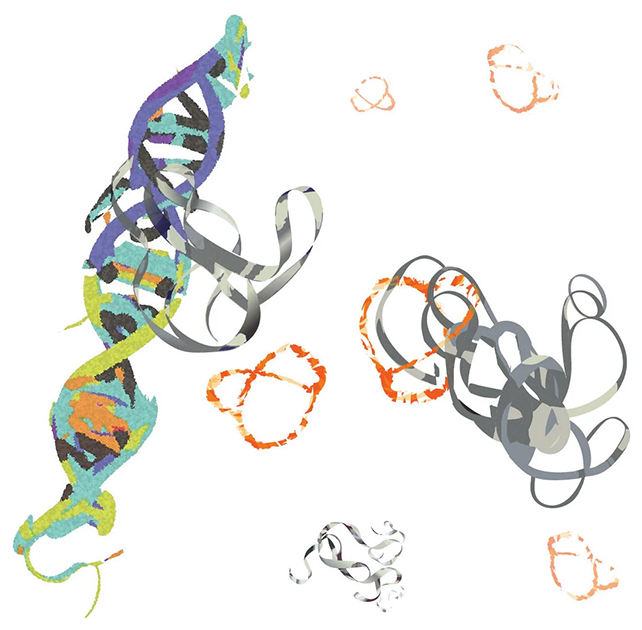 The peptide (orange) binds to MYC (gray) to stop overactive DNA interactions. (Min Xue/UCR)
The peptide (orange) binds to MYC (gray) to stop overactive DNA interactions. (Min Xue/UCR)"MYC is less like food for cancer cells and more like a steroid that promotes cancer's rapid growth," says biochemist Min Xue, from UCR. "That is why MYC is a culprit in 75 percent of all human cancer cases."
"Normally, MYC's activity is strictly controlled. In cancer cells, it becomes hyperactive, and is not regulated properly."
The researchers were able to study the small amounts of structure that MYC does have in order to build up a library of peptides that might be able to snag on to that structure. One peptide in particular, NT-B2R, proved particularly adept at disabling MYC.
In tests using a culture made from human brain cancer cells, NT-B2R was shown to successfully bind to MYC, changing the way cells regulated many of its genes and ultimately decreasing the metabolism and proliferation of the cancer cells. It's a bit like tying someone's hands behind their back, stopping them from doing anything much at all.
Key to the breakthrough was earlier work by some of the same researchers, which recognized that as the structure and shape of peptides were changed, these molecules became better at interacting with shapeless proteins – like MYC.
"Peptides can assume a variety of forms, shapes, and positions. Once you bend and connect them to form rings, they cannot adopt other possible forms, so they then have a low level of randomness. This helps with the binding," says Xue.
"We improved the binding performance of this peptide over previous versions by two orders of magnitude. This makes it closer to our drug development goals."
There's still a lot of work to be done, though these early results are promising. Right now the peptide is being delivered via fatty spheres called lipid nanoparticles, which aren't really suitable for dispensing drugs – so that will need to be changed.
Rigorous tests in human subjects will also need to be carried out, but we just might have found a method for stopping one of the ways in which cancer hijacks healthy biological processes in order to survive.
"MYC represents chaos, basically, because it lacks structure," says Xue.
"That, and its direct impact on so many types of cancer make it one of the holy grails of cancer drug development. We are very excited that it is now within our grasp."
The research has been published in the Journal of the American Chemical Society.

 11 months ago
69
11 months ago
69