ARTICLE AD
Accidental overdoses from 'copies' of the weight-loss drug sold commercially as Ozempic and Wegovy are on a steep rise in the US. So much so, the Food and Drug Administration (FDA) just had to issue a warning to patients, health providers, and compounding pharmacies.
According to a new public alert, government health officials have received a worrying number of dosing mistakes related to injectable semaglutide – the active ingredient in Ozempic and Wegovy.
Symptoms can include nausea, vomiting, fainting, migraine, dehydration, pancreatitis, and gallstones. Sometimes, overdoses can lead to hospitalization.
At the end of last year, America's Poison Centers said they received 15 times more calls related to semaglutide than they did in 2019.
Officials at the FDA say compounded drugs put patients at higher risk for overdose.
Semaglutide injections were originally approved by the FDA to treat diabetes, but in recent years, they have exploded in use due to their rapid weight loss effects. Between 2019 and the end of 2023, prescriptions for semaglutide medications increased by 932 percent.
The burst in popularity led to a shortage in FDA-approved injections, leaving the market open to unapproved 'copies' of the product, called compounded drugs.
When FDA-approved, semaglutide injections are only available in pre-filled pens. But compounded semaglutide is mixed by licensed pharmacists or at drug facilities with differing concentrations from vial to vial.
Some compounders instruct patients to administer semaglutide in 'units', while others indicate dosages in milligrams or milliliters. Many provide large syringes that were not intended to be used for small doses of semaglutide.
"The majority of the reports described patients mistakenly drawing up more than the prescribed dose from a multiple-dose vial during self-administration," officials at the FDA explain.
"In these instances, patients administered five to 20 times more than the intended dose of semaglutide. Most of the reports indicated that patients were unfamiliar with how to measure the intended dose using a syringe."
In one case reported to the FDA, a provider intended to dose 0.25 milligrams of semaglutide, but instead gave their patient 25 units – five times the correct amount. This led to the patient experiencing severe vomiting.
In another case, a provider prescribed 20 units instead of 2 units. Three of their patients experienced nausea and vomiting as a result.
And when some patients were instructed to administer just 5 units of semaglutide from a 100-unit syringe, they accidentally gave themselves 50 units – ten times what was intended.
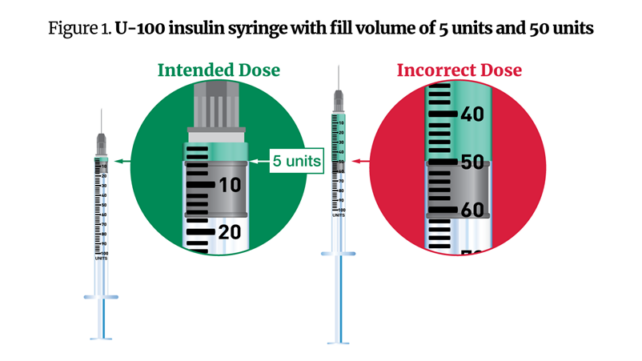 Semaglutide dosing error showing intended dose versus incorrect dose. (FDA)
Semaglutide dosing error showing intended dose versus incorrect dose. (FDA)To avoid these mistakes, the FDA advises health care providers and compounders to give patients appropriately sized syringes that cannot be filled so far beyond the intended dosage.
Patients should also be carefully instructed on how to measure the intended dosage using a syringe, and health care providers should be vigilant in their dosage conversions when prescribing compounded drugs.
If an overdose from semaglutide is ever suspected, officials say medical attention should be sought immediately.

 3 months ago
31
3 months ago
31