ARTICLE AD
Early screening for neurodevelopmental disorders such as autism is important to ensure children have the support they need to gain the essential skills for daily life.
The American Academy of Pediatrics recommends that all children be screened for developmental delays, with additional screening for those who are preterm or have a low birth weight.
However, the US Preventive Services Task Force has called for more research into the effectiveness of current autism screening practices.
Primarily based on milestone checklists and symptoms, autism diagnoses also currently rely on observations of behavior that often manifests after crucial developmental stages have passed.
Researchers and clinicians are working to develop simple, reliable tools that could identify early signs or risk factors of a condition before symptoms are obvious.
While early screening can lead to the risk of overdiagnosis, understanding a child's developmental needs can help guide families toward resources that address those needs sooner.
We are researchers who study the role the microbiome plays in a variety of conditions, such as mental illness, autoimmunity, obesity, preterm birth and others.
In our recently published research on Swedish children, we found that microbes and the metabolites they produce in the guts of infants – both found in poop and cord blood – could help screen for a child's risk of neurodevelopmental conditions such as autism.
And these differences can be detected as early as birth or within the first year of life. These markers were evident, on average, over a decade before the children were diagnosed.
Microbes as biomarkers
Biomarkers are biological indicators – such as genes, proteins or metabolites in blood, stool or other types of samples – that signal the presence of a condition at a certain point in time.
There are no known biomarkers for autism. Efforts to find biomarkers have been largely hindered by the fact that autism has many potential pathways that lead to it, and researchers tend to ignore how these causes may work together as a whole.
One potential biomarker for neurodevelopmental conditions such as autism are gut microbes. The connection between the gut and brain, or the gut-brain axis, is an area of considerable interest among scientists. Gut microbes play significant roles in health, including in immunity, neurotransmitter balance, digestive health and much more.
A lot of work has been done around mapping what a "typical" microbiome looks like based on age and organ system. Researchers have shown that the microbiome is personalized enough that it can distinguish two people or two households even better than genetics, with differences in colonization starting very early in life.
The microbiome undergoes immense changes during childhood. It shapes and is shaped by the immune system and influenced by life changes and events. It is also influenced by factors like genetics, environment, lifestyle, infection and medications.
Gastrointestinal symptoms such as diarrhea, pain and constipation are common in children with autism and ADHD, with as many as 30% to 70% of autism patients also diagnosed with functional gastrointestinal disorders.
Untreated GI issues can also lead to additional sleep and behavioral disorders among these children.
A small pilot study found that children with autism showed improvements in gastrointestinal and autism-related symptoms after having healthy microbes transferred into their guts, with some benefits lasting up to two years.
allowfullscreen="allowfullscreen" frameborder="0″>
Your gut and your brain are intricately connected.Most studies on the microbiome and neurodevelopmental conditions, however, are restricted to people who are already diagnosed with ADHD, autism or other conditions, and these studies often show mixed results.
These limitations raise an important question: Does the microbiome play a direct role in the development of autism and other neurodevelopmental conditions, or are changes in microbiome composition a consequence of the conditions themselves?
Some investigations have proposed that the microbiome has little or no association with future autism.
However, these studies have a notable limitation: They don't examine microbial imbalances prior to diagnosis or symptom onset. Instead, these studies focus on children already diagnosed with autism, comparing them to their siblings and unrelated neurotypical children.
In most cases, dietary data and samples are collected several years after diagnosis, meaning the study cannot test for whether microbial imbalances cause autism.
Microbes matter
We wondered whether studying the bacteria residing in small children before they are diagnosed or show symptoms of autism or other conditions could give us a clue into their neurodevelopment.
So, we examined the cord blood and stool collected at approximately 1 year of age from participants of an ongoing study called All Babies in Southeast Sweden, which follows the health of approximately 17,000 children born between 1997 and 1999 and their parents.
We have followed these children since birth, nearly 1,200 of whom were later diagnosed with a neurodevelopmental disorder by age 23.
We found significant differences in bacterial composition and metabolite levels that developed before symptoms of neurodevelopmental conditions – such as gastrointestinal upset, crankiness and sleep problems – as well as formal medical diagnoses. These differences spanned many conditions, including autism, ADHD and speech disorders.
Next, we linked bacteria to neurotransmitters – chemical signals that help brain cells communicate – and vitamins such as riboflavin and vitamin B in the child's stool.
Given previous research on children and adults already diagnosed with a neurodevelopmental disorder, we expected to find differences in the microbiome composition and health between those with and without neurodevelopmental conditions.
But we were surprised to discover just how early these differences emerge. We saw variability in the microbes and metabolites that affect immune and brain health, among others, in the stool collected from the diapers of children around 1 year of age and in umbilical cord blood collected at birth.
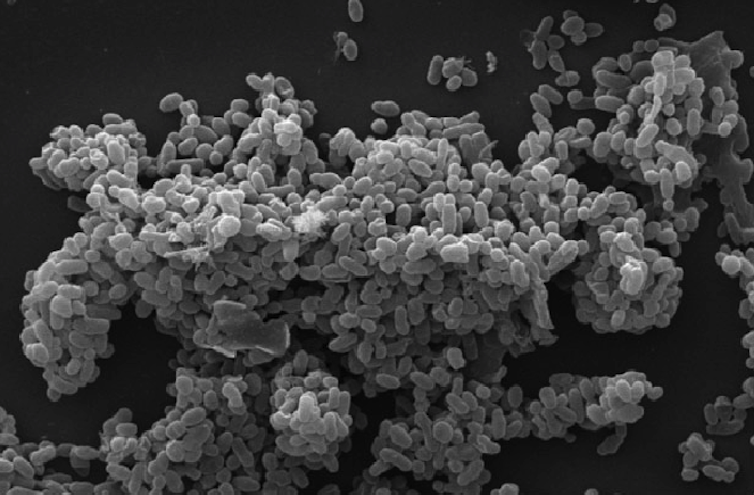 The researchers identified a link between imbalance of Akkermansia muciniphila and later development of neurodevelopmental disorders. (Zhang et al. 2019/Microbial Biotechnology/CC BY-SA)
The researchers identified a link between imbalance of Akkermansia muciniphila and later development of neurodevelopmental disorders. (Zhang et al. 2019/Microbial Biotechnology/CC BY-SA)The imbalance in microbial composition – what microbiologists call dysbiosis – we observed suggests that incomplete recovery from repeated antibiotic use may greatly affect children during this vulnerable period. Similarly, we saw that repeated ear infections were linked to a twofold increased likelihood of developing autism.
Children who both repeatedly used antibiotics and had microbial imbalances were significantly more likely to develop autism.
More specifically, children with an absence of Coprococcus comes, a bacterium linked to mental health and quality of life, and increased prevalence of Citrobacter, a bacterium known for antimicrobial resistance, along with repeated antibiotic use were two to four times more likely to develop a neurodevelopmental disorder.
Antibiotics are necessary for treating certain bacterial infections in children, and we emphasize that our findings do not suggest avoiding their use altogether.
Parents should use antibiotics if they are prescribed and deemed necessary by their pediatrician. Rather, our study suggests that repeated antibiotic use during early childhood may signal underlying immune dysfunction or disrupted brain development, which can be influenced by the gut microbiome.
In any case, it is important to consider whether children could benefit from treatments to restore their gut microbes after taking antibiotics, an area we are actively studying.
Another microbial imbalance in children who later were diagnosed with neurodevelopmental disorders was a decrease in Akkermansia muciniphila, a bacterium that reinforces the lining of the gut and is linked to neurotransmitters important to neurological health.
Even after we accounted for factors that could influence gut microbe composition, such as how the baby was delivered and breastfeeding, the relationship between imbalanced bacteria and future diagnosis persisted.
And these imbalances preceded diagnosis of autism, ADHD or intellectual disability by 13 to 14 years on average, refuting the assumption that gut microbe imbalances arise from diet.
We found that lipids and bile acids were depleted in the cord blood of newborns with future autism. These compounds provide nutrients for beneficial bacteria, help maintain immune balance and influence neurotransmitter systems and signaling pathways in the brain.
Microbiome screening at well-child visits
Microbiome screening is not a common practice in well-child visits. But our findings suggest that detecting imbalances in beneficial and harmful bacteria, especially during critical periods of early childhood development, can provide essential insights for clinicians and families.
There is a long way to go before such screening becomes a standard part of pediatric care. Researchers still need validated methods to analyze and interpret microbiome data in the clinic.
It's also unclear how bacterial differences change across time in children around the world – not just which bacteria are present or absent, but also how they may be shaping immune responses and metabolism.
But our findings reaffirm the growing body of evidence that the early gut microbiome plays a key role in shaping neurodevelopment.![]()
Angelica P. Ahrens, Assistant Research Scientist in Data Science and Microbiology, University of Florida; Eric W. Triplett, Professor and Chair of Microbiology and Cell Science, University of Florida, and Johnny Ludvigsson, Professor Emeritus of Biomedical and Clinical Sciences, Linköping University
This article is republished from The Conversation under a Creative Commons license. Read the original article.

 1 month ago
19
1 month ago
19 

