ARTICLE AD
 Artist's impression of glial cells. (Artur Plawgo/Science Photo Library/Getty Images)
Artist's impression of glial cells. (Artur Plawgo/Science Photo Library/Getty Images)
Tangles of tau proteins in the brain are a hallmark of Alzheimer's disease, but recently, these crucial filaments have acquired a 'bad guy' reputation they do not fully deserve.
Like the story of Jekyll and Hyde, the tale of tau is a complicated mix of good and bad.
Surprising new research on the brain cells of flies and rats suggests tau proteins usually protect the brain from degeneration. Only when they are absent or defective do they appear to contribute to brain disease.
"By revealing a surprising new neuroprotective role for tau, the study opens the door to potential new strategies to slow, reverse, and treat neurodegenerative conditions," says neuroscientist Hugo Bellen from the Baylor College of Medicine in the US.
The findings support a potential root cause of Alzheimer's disease, which is centered around fat droplets in the brain's connective tissue.
These fat droplets are sort of like garbage bags for neurological waste. When a neuron needs to rid itself of toxic materials, like reactive oxygen species (ROS), it exports its waste to neighboring cells, called glia, which then package the ROS into lipid droplets.
"This process effectively removes and neutralizes these toxic lipids," explains Lindsey Goodman, a neurobiologist at Bellen's lab.
When tau proteins are absent from the glia of flies, however, these lipid droplets cannot form, and toxic ROS accumulates in the brain, leading to motor defects.
Goodman and her colleagues found that even a 50 percent loss of healthy tau is sufficient to disrupt lipid droplets and contribute to toxicity in the brains of flies.
A similar effect is seen in glial cells from rats and humans, which suggests tau also plays a neuroprotective role in mammals like ourselves.
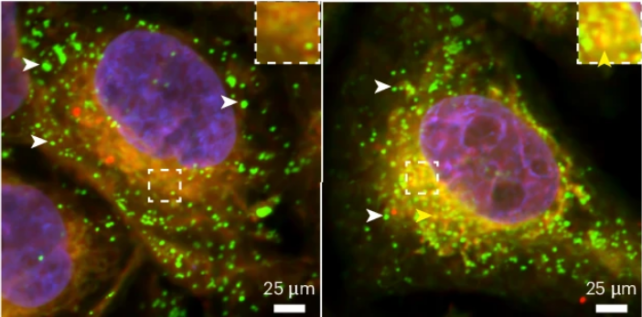 Tau loss disrupts budding of lipid droplets . White arrowheads point to mature lipid droplets that have budded. Yellow arrowheads point to future lipid droplets that still need to bud from the glial cell. (Goodman et al., Nature Neuroscience, 2024)
Tau loss disrupts budding of lipid droplets . White arrowheads point to mature lipid droplets that have budded. Yellow arrowheads point to future lipid droplets that still need to bud from the glial cell. (Goodman et al., Nature Neuroscience, 2024)The findings hint at a whole new benefit of tau. These proteins are abundant in the human brain, and when healthy, they fold and bind together to create an internal 'skeleton' for brain cells.
Problems start to occur, however, when tau is missing or mutated, and its folding goes awry.
In a 'humanized' fly model with the same tau mutations seen in some cases of Alzheimer's disease, researchers found tau proteins could no longer combat rising ROS levels.
When ROS was increased in experiments, the flies with mutated tau showed significant damage to their glial cells.
The study provides "compelling evidence" that tau loss is only detrimental in the presence of ROS, the authors say. This potentially explains why mice that are genetically programmed to lack tau do not show signs of neurodegeneration until old age, when ROS levels begin to accumulate.
In other words, it's not just a lack of healthy tau that can cause problems. It's how that lack of healthy Tau interacts with a spike in toxic brain byproducts.
"While low levels of ROS are beneficial, excess ROS is harmful to cells as it triggers the production of toxic forms of other molecules that induce oxidative stress," explains Goodman.
In recent years, misfolded tau proteins have emerged as one of the earliest biomarkers of Alzheimer's disease, capable of damaging or killing neurons over time. But scientists are still trying to figure out if these tangles are a root cause of neurodegeneration, or a response to some other underlying issue.
While tangles of Tau are found in some brains of those who die with Alzheimer's, the mutated proteins cannot fully explain all the different subtypes of this neurodegenerative disease.
In the last 17 years, more than 30 drugs that target tau have reached clinical trials after showing promise in animal models, and yet to date none have produced benefits in human patients.
The new research suggests that a blanket attack on all forms of tau may be eliminating the 'good' players along with the 'bad'.
When it comes to tau-associated brain disease, nuance appears to be key.
The study was published in Nature Neuroscience.

 2 months ago
27
2 months ago
27 

