ARTICLE AD
Scientists have identified the reward pathway that when shared by drugs like cocaine and morphine disrupts our natural desire for more life-sustaining needs like food and water.
The discovery helps explain why addictive drug use can override the importance of even eating and drinking, and it could lead to new treatment interventions.
A New York team from Rockefeller University and the Icahn School of Medicine at Mount Sinai used mouse models to study responses to morphine and cocaine in brain reward circuits usually activated by hunger and thirst.
"We've known for decades that natural rewards, like food, and drugs can activate the same brain region. But what we've just learned is that they impact neural activity in strikingly different ways," says Rockefeller University neuroscientist Jeffrey Friedman.
"One of the big takeaways here is that addictive drugs have pathologic effects on these neural pathways, that's distinct from, say, the physiologic response to eating a meal when you are hungry or drinking a glass of water when you are thirsty."
We started the project by asking a simple question: how does the development of drug addiction interfere with our natural needs? This led us to collaborate across multiple fields to unravel the underlying mechanisms. I am truly grateful to work with these hardcore scientists! https://t.co/ffIX2ZHEHP
— Bowen Tan (@tbw_owl) April 19, 2024The team used a whole-brain approach – mapping brain activity, imaging neurons in action in living mice, and sequencing the genetic activity of individual cells altered with CRISPR to see how cocaine and morphine might 'hijack' their natural reward pathways.
They found that the brain's nucleus accumbens (NAc) is more crucial than we thought for both typical functions and drug rewards. Neurons projecting to the NAc from the brain's orbitofrontal cortex appear to be the culprits that reduce our desire for natural rewards when activated by drug use.
In conjunction with dopamine and serotonin, the NAc uses motivation, positive reinforcement, and pleasure to help us keep doing things that make us feel good.
"The NAc is a key node where the underlying dopaminoceptive neurons direct and refine animals' behaviors towards their goals," says neuroscientist Bowen Tan, a graduate student at Rockefeller University during the study.
"What we hadn't been able to understand is how repeated exposure to drugs corrupts these neurons, resulting in escalated drug-seeking behaviors and a shift away from healthy goals."
frameborder="0″ allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share" referrerpolicy="strict-origin-when-cross-origin" allowfullscreen>
Cocaine – a central nervous system stimulant and one of the most addictive substances we know of – has different effects on the brain to morphine, an opioid that provides pain relief.
The researchers discovered that cocaine and morphine each activate a specific subset of neurons in the mouse NAc, most of which overlap with neurons that respond to natural rewards. But these overlapping neurons became more active when mice were given cocaine or morphine compared to when they were given food or water.
Repeated exposure to the drugs gradually changed the mice's behavior. They became more interested in cocaine and morphine, and less interested in plain old food and water, which elicited the same baseline level of response every time.
"By tracking these cells, we show that not only are similar cells activated across reward classes, but also that cocaine and morphine elicit initially stronger responses than food or water, and this actually magnifies with increasing exposure," explains neuroscientist Caleb Browne from Icahn School of Medicine at Mount Sinai.
"After withdrawal from the drugs, these same cells exhibit disorganized responses to natural rewards in a manner that may resemble some of the negative affective states seen in withdrawal in substance use disorder."
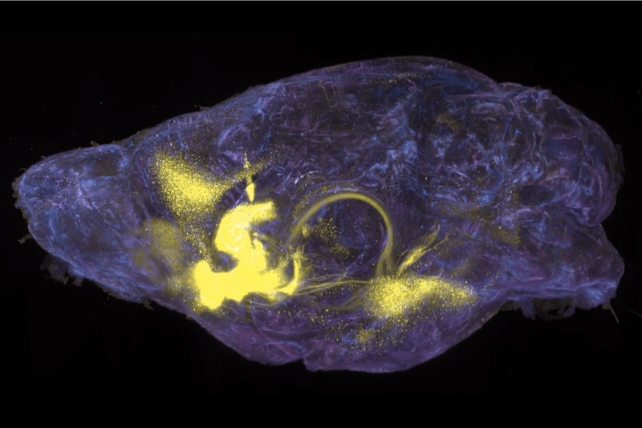 Brain-wide mapping of drug-activated neurons that project to a mouse's NAc. (Rockefeller University)
Brain-wide mapping of drug-activated neurons that project to a mouse's NAc. (Rockefeller University)They also found a protein encoded by the Rheb gene that plays a critical role in interfering with typical neuron communication, changing how the brain 'remembers' rewards from food and water. Pathways associated with this particular protein could be a target for therapy.
Understanding how addictive drugs can mess with a very well-coordinated system that normally connects physiological needs to appetite-related behavior, could mean we find better ways to manage addiction, which currently has few effective treatments.
"Ongoing research will be directed to defining how the flow of multimodal information is incorporated into value computations in brain cells and how that crucial mechanism enables drugs to overtake the processing of natural rewards, leading to addiction," says Mount Sinai neuroscientist Eric Nestler.
The research has been published in Science.

 7 months ago
45
7 months ago
45