ARTICLE AD
In 1896, German chemist Emil Fischer noted something very strange about a molecule named acetaldehyde phenylhydrazone. Identical batches of the crystalline compound appeared to have wildly different melting points.
Some batches, he found, melted at temperatures of around 65 degrees Celsius (149 Fahrenheit). Others at 100 degrees Celsius.
It was, in a word, utterly bizarre. No other substance was known to behave this way. Nor should it. According to the laws of thermodynamics that describe the way the physical world behaves, such a result should be impossible.
Scientists were stumped. They rushed to see if Fischer had made a mistake. Imagine their consternation when they were able to replicate his observations.
More than 120 years after Fischer's original discovery, in 2019, an international team of researchers led by chemist Terry Threfall of the University of Southampton in the UK finally found and published the answer. Fischer (who went on to win a 1902 Nobel prize for other work, so he was clearly no quack) had observed something real; but not, as it would turn out, anything that would break thermodynamics.
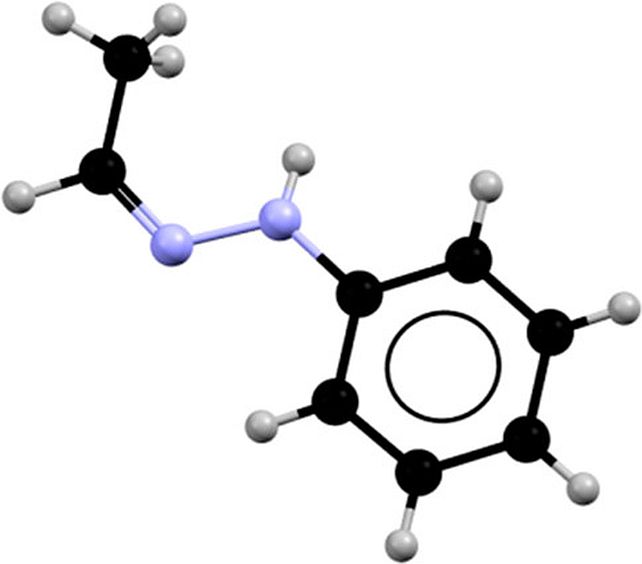 The structure of acetaldehyde phenylhydrazone. (Bernades et al., Cryst. Growth Des., 2019)
The structure of acetaldehyde phenylhydrazone. (Bernades et al., Cryst. Growth Des., 2019)The culprit? An absolutely miniscule contamination, so small that it is all but undetectable. When acetaldehyde phenylhydrazone melts, it becomes one of two liquids, based on whether the compound has been exposed to a base or an acid. The former appears at the higher melting point; and the latter at the lower.
"It is just exceedingly satisfying to be able to understand such an ancient puzzle, especially one which baffled such an eminent scientist who became a Nobel Prize winner," Threlfall said.
"The observation of such behavior will be exceedingly rare because it depends on the molecules in the crystal and in the liquid having different geometries, which is unusual. Furthermore, it depends also on the conversion by acid being both possible and rapid."
The compound is made by dissolving solid acetaldehyde and adding both liquid phenylhydrazine and aqueous ethanol, and chilling until the mixture freezes and forms solid crystals. To then discover the melting point of the newly formed acetaldehyde phenylhydrazone, you have to re-melt it.
This is where the problems emerged. To understand why acetaldehyde phenylhydrazone melts at two distinct temperatures, the researchers first investigated its solid form. But the most cutting edge probes failed to turn up an answer.
All analyses, performed by Threlfall's team and other recent efforts, failed to find a single difference between acetaldehyde phenylhydrazone samples that melted at the lower temperature, and samples that melted at the higher. These techniques included X-ray diffraction, nuclear magnetic resonance, and IR spectroscopy. As far as scientists could tell, the crystals were identical.
The next step was to investigate the liquid the crystals became after melting.
And there, the researchers got a result. There was a subtle, and temporary, but distinct difference. Although the compounds had the same molecular formula, the structure of the initial melt was slightly different, depending on the temperature.
The compound contains a methyl group that is able to have two distinct configurations, known as the Z isomer and the E isomer.
In its solid phase, the material almost exclusively consists of the Z isomer.
The most stable liquid phase is a mix of about one-third Z isomer to two-thirds E isomer. The lower of the two melting points immediately produces the Z and E mix, while the higher melting point is entirely Z, before switching to part E.
A clue was given in a 1905 paper, which pointed out that acetaldehyde phenylhydrazone was extremely sensitive to acid. Threlfall and his team tried exposing their samples to vapors of acid and ammonia. And they found that exposure to just a tiny bit of one or the other could reliably influence the compound's melting point. The acid acts as a catalyst to speed the shift from the Z to E isomer, lowering the melting point in the process.
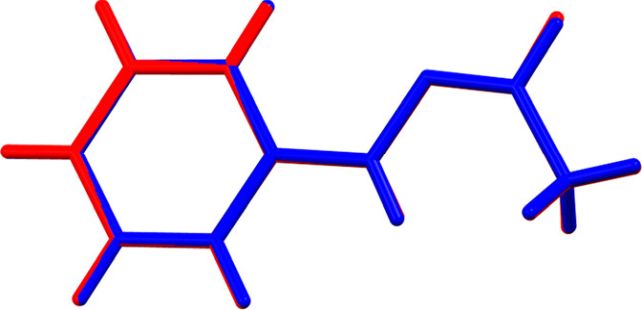 Molecular structure overlap of high-melting (blue) and low-melting (red) forms of acetaldehyde phenylhydrazone. (Bernades et al., Cryst. Growth Des., 2019)
Molecular structure overlap of high-melting (blue) and low-melting (red) forms of acetaldehyde phenylhydrazone. (Bernades et al., Cryst. Growth Des., 2019)"If an element or compound can exist in two or more distinct crystalline forms, then each form will have different Gibbs energies and melt at its own distinct temperature," said chemist Simon Coles of the University of Southampton.
"In this case, the molecules of the crystal are in the cis geometry – of groups pointing towards each other – and melt to an identical geometry in the absence of acid at 100 degrees Celsius. However, in the presence of even a trace of acid, the molecules convert on melting to the trans geometry of groups pointing away from each other. This liquid has a smaller Gibbs energy and is more stable, so the melting point becomes 65 degrees Celsius."
It's similar to the effect salt has on water: adding salt to a pot of water raises the freezing and boiling points. Where it takes a lot of salt to invoke a significant change to water's phase transitions, it takes so little acid to alter acetaldehyde phenylhydrazone that it took more than a century – and Threlfall and his colleagues a decade – to figure it out.
This research is a real testament to human curiosity and tenacity. And it gives us hope for the future. How many more mysteries will be solved in the years stretching into a glittering future of discovery?
The research was published in 2019 in Crystal Growth & Design.

 3 days ago
7
3 days ago
7 
