ARTICLE AD
Given the right conditions, tiny airborne particles of the rock mineral feldspar can influence cloud formation. Just how this happens, however, has never been clear.
Now new research has now solved the mystery of how this process actually works.
It's previously been established that this ubiquitous material – which makes up half of Earth's crust and can also be found on other planets – is attractive to water molecules, making it a good nucleation seed for vapor.
As water molecules attach to feldspar dust high in the atmosphere and start freezing, the seeds of clouds are sown. In this new research, a team from the Vienna University of Technology (TU Wein) in Austria used a highly sensitive atomic force microscope to take a closer look.
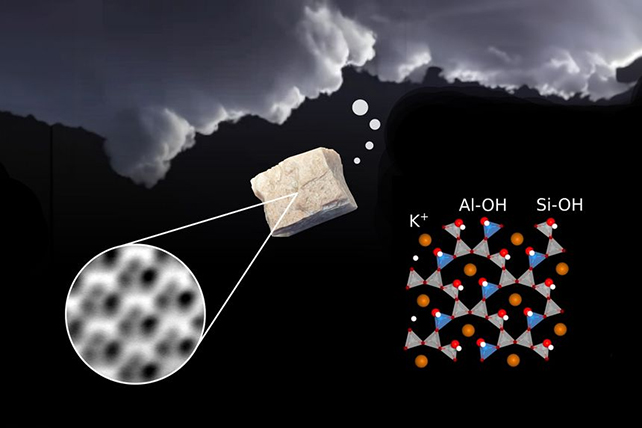 Scientists took a very close look at feldspar. (TU Wein)
Scientists took a very close look at feldspar. (TU Wein)"We placed a piece of feldspar in the microscope's vacuum chamber and split it in half to obtain a pristine and clean surface," says TU Wein physicist Giada Franceschi. "We were puzzled by the results: the images of the surface looked different from what common theories had predicted."
The peculiar geometry of the feldspar surface revealed by the ultra-high resolution images was found to be caused by tiny pockets of water called inclusions. It turns out that when the rock gets split, a small amount of water vapor gets released from these pockets, which then attaches back to the surface.
This attachment and the energy released by the splitting of the rock causes the water molecules themselves to break apart, creating what are known as hydroxyl groups (OH) – single oxygen and hydrogen atoms linked together.
And it's these hydroxyl groups that are key to the close attraction between water and feldspar: as the researchers were able to confirm by running computer simulations of the chemical reactions, the hydroxyl groups are perfect anchor points for water molecules to attach themselves to.
"The bond is established very easily and quickly, and it is also very stable," says physicist Ulrike Diebold, from TU Wein.
"To remove the hydroxyl layer from feldspar, one would have to heat it to a high temperature."
Feldspar is an important material for Earth's carbon and potassium cycles as well as the water cycle, and understanding more about how it interacts with other elements will teach us more about those cycles too.
When it comes to cloud formation, it's vital that we understand how climate change is going to affect the atmosphere and the clouds in it – another area where this study will prove useful in the future.
For now, it solves one of the mysteries of feldspar that had researchers puzzled. Previous hypotheses had considered the effects of the potassium atoms in the rock, and the defects in its crystal structure.
"Researchers were considering several ideas why feldspar is such an effective nucleation seed," says Diebold.
The research has been published in the Journal of Physical Chemistry Letters.

 1 year ago
109
1 year ago
109